Osmotic Fragility Test Of Erythrocyte
INTRODUCTION
Red blood cell membrane restricts the solutes and allows only water to pass through them(osmosis). if the cells are placed in hypertonic solution sodium chloride (solution concentrated than normal saline e.g. 2%, (W/V), they shrink due to exosmosis. The red cells absorb water when kept in hypertonic solution (solution concentration less than even 0.55% (w/v) saline). Due to endosmosis, hemolysis of the takes place due to the excessive swelling. In isotonic solution (normal saline), however, the red cells stay intact.
CLINICAL SIGNIFICANCE
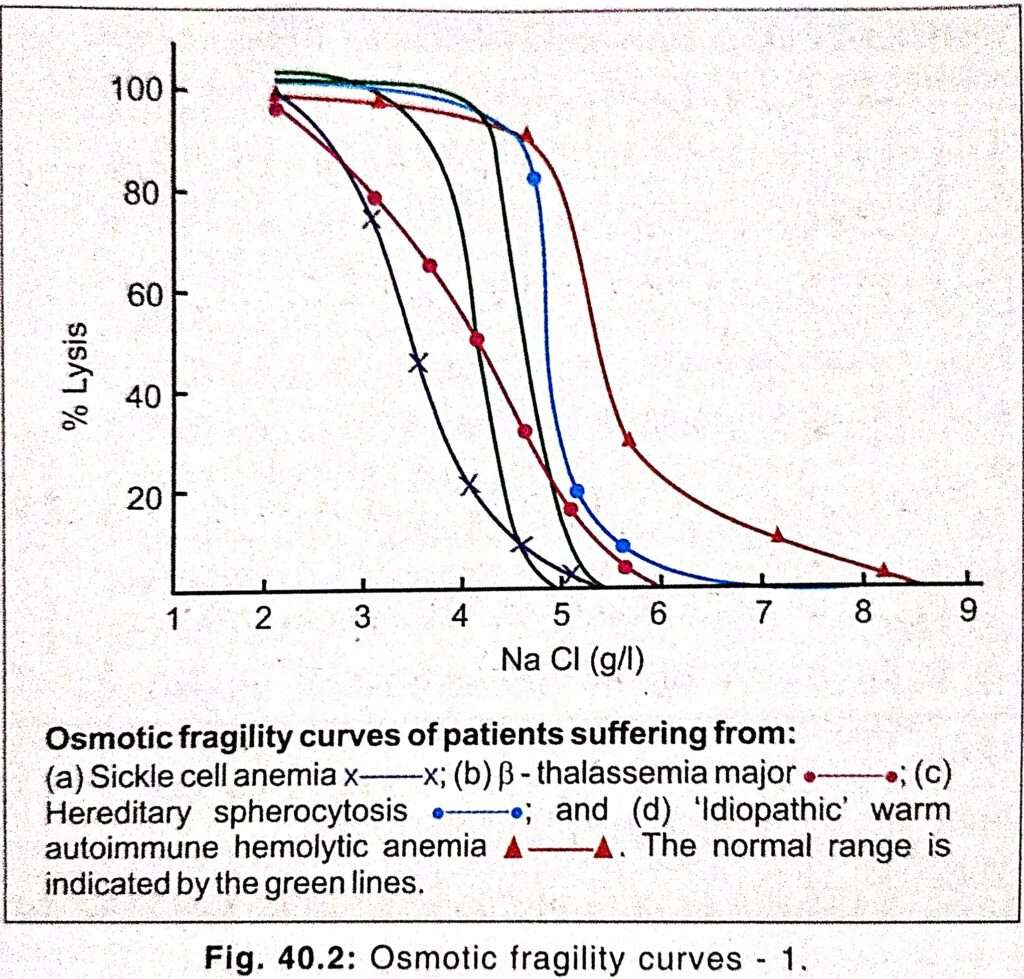
In conditions such as congenital spherocytosis the red cells are highly fragile and start to hemolyze at 0.5% (w/v) sodium chloride while normal cells start to hemolyze at 0.45% (w/v) of sodium chloride. Thalassemic target cells hemolyze below 0.3% sodium chloride.
SPECIMEN
- Heparinized blood
- Defibrinated blood
For the type of specimen, about 10 ml of blood is collected in a conical flask containing 10-15 glass beads. After rotating the blood gently for 5 minutes it is centrifuged to gel red blood.
PRINCIPLES
The red cells are suspended in decreasing concentration of sodium chloride, ranging from 0.9% (w/v) to 0.0% (water). Degree of hemoysis is measured in each tube by using, a photometer.
REQUIREMENTS
- Test tubes
- Pipettes
- Photometer
- Graph papers
REAGENTS
- 10 g/dl, stock buffered sodium chloride
- Sodium chloride : 90 g
- Na2HPO4 (Anhydrous) : 13.65 g
- NaH2PO4 (Anhydrous) : 2.43 g
- Distilled Water : 1000 ml
- Prepare 1 g/dl of buffered solution by mixing 10 ml of solution 1 and 90 ml of Distilled water.
- Now prepare working solutions in centrifuge tubes as follows:
| Working solution No. | 1g/dl, buffered saline, ml | Distilled water, ml | Concentration of working solutions, g/dl |
| 3 | 2.7 | 6.3 | 0.3 |
| 4 | 3.15 | 5.85 | 0.35 |
| 5 | 3.6 | 5.4 | 0.40 |
| 6 | 4.05 | 4.95 | 0.45 |
| 7 | 4.5 | 4.5 | 0.50 |
| 8 | 4.95 | 4.05 | 0.55 |
| 9 | 5.4 | 3.6 | 0.60 |
| 10 | 6.3 | 2.7 | 0.70 |
| 11 (BLANK) | 7.2 | 1.8 | 0.80 |
| 12 | 0.0 | 9.0 | 0.0 |
Tube No.11 (blank) gives 0% hemolysis.
Tube No.12 gives 100% hemolysis.
Preparation of. 50% suspension of patients red cells in 1 g/dl, buffered saline.
- Take about 5 ml of blood in a graduated centrifuge tube.
- Centrifuge at. 1500 RPM for 10 minutes.
- Discard the supernatant and add 2 to 3 ML of 1 g/dl, buffered saline mix and centrifuge at 1500 rpm for 5 minutes.
- Discard the supernatant and repeat Step 3 at least 3 times. discard the supernatant.
- Measure the volume of packed cells.
- Add equal quantity of 1 g/dl buffered saline and mix well.
PROCEDURE
- Add 1 ml of 50% cell suspension to each of the 12 working solutions of buffered saline (prepared at steps-C.
- Mix well and keep all the tubes at room temperature for 30 minutes.
- Mix again and centrifuge at 1500 pm for 10 minutes.
- Read absorbance of the supernatant of each tube at 540 nm (green filter) by setting blank (tube no. 11) to 100% T.
- Use following formula to calculate percent hemolysis.
- Percent hemolysis = O.D. of individual tube × 100 / *Absorbance with 100% hemolysis
- *Absorbance with 100% hemolysis is obtained in tube No. 12.
- Draw the fragility curve by plotting percent hemolysis on Y axis. And concentration of buffered saline on X axis.
- Determine median corpuscular fragility (MCF) which causes 50% lysis.
MAIN REQUIREMENTS
Specimen : Defibrinated blood
Container : Screw-capped sterile bottles of 5 ml (two)
METHOD
- Incubate to ml of blood in sterile 5 ml screw capped bottles at 37 °C.
- After 24 hours perform osmotic fragility test as described earlier.
- Draw fragility curves.
Following factors affect osmotic fragility tests.
- The relative volume of blood and saline.
- The final page of the blood in saline suspension.
- The temperature at which the tests are carried out.
- Thappad saline is used to control the final pH of the blood in saline suspension.
- 50% cells suspension is used to avoid interference of plasma.
- The median corpuscular fragility (MCF) i.e. concentration of saline causing 50% lysis may also be determined.
- Osmatic fragility in health (at 20 °C and pH 7.4).
| Fresh blood (g/l Nacl) | Blood incubated for 24 hrs. at 37 °C (g/l/Nacl) | |
| Initial lysis | 5.0 | 7.0 |
| Complete lysis | 3.0 | 2.0 |
| MCF (50% lysis) | 4.0-4.45 | 4.65-5.9 |
- The osmotic fragility of freshly taken red cells reflects their ability to take up a certain amount of water before lysing.
- The ability of the normal cells to withstand hypotonicity results from its biconcave shapes which allows the cells to increase it’s volume by about 70% before the surface membrane is stretched. Once this limit is reached lysis occurs.
- Spherocytes have an increased volume to surface area ratio. They are susceptible to osmotic lysis.
- Decreased osmotic fragility indicates presence of unusually flattened red cells in which the volume to surface area ratio is decreased. such a change occur in iron deficiency anemia and thalassemia, in which the red cells with a low MCH and MCV are usually resistant to osmotic lysis.
- The increased osmotic fragility of normal red cells which occurs after incubation is mainly caused by swelling of the cells associated with an accumulation of sodium which exceeds loss of potassium.
- The osmotic fragility of red cells which have an abnormal membrane, such as those of HS (Hereditary spherocytosis) and HE (hereditary elliptocytosis), increase abnormality after incubation.
- In thalassemia minor and major, osmotic fragility is frequently markedly reduced after incubation (due to loss of potassium ions).
IMPORTANT IMFORMATION
- In the case of a normal person the MCF is close to 0.45% or slightly lower.
- MCF is about 0.5% in hereditary spherocytosis.
- MCF decreases is sickle cell anemia and in thalassemia (0.35% to 0.4%).
- As a part of quality control a parallel run should be done with a normal blood specimen.
Preparation of Lupus Erythematosus (LE) Cell
IMPORTANT MCQS OF OSMOTIC FRAGILITY TEST OE ERYTHROCYTE
What is the purpose of the osmotic fragility test?
- a) To measure the volume of red blood cells
- b) To measure the ability of red blood cells to maintain their shape in varying concentrations of salt
- c) To measure the level of hemoglobin in the blood
- d) To measure the ability of red blood cells to clot
Answer: b) To measure the ability of red blood cells to maintain their shape in varying concentrations of salt
Which of the following is true regarding the osmotic fragility test?
- a) It is a test used to diagnose anemia
- b) It is a test used to diagnose blood disorders
- c) It is a test used to diagnose infections
- d) It is a test used to diagnose cardiovascular diseases
Answer: b) It is a test used to diagnose blood disorders
In the osmotic fragility test, what happens to the red blood cells when they are placed in a solution of increasing salt concentration?
- a) They expand and burst
- b) They shrink and become more rigid
- c) They expand and become more flexible
- d) They shrink and burst
Answer: d) They shrink and burst
Which of the following diseases can be diagnosed using the osmotic fragility test?
- a) Sickle cell anemia
- b) Thalassemia
- c) Hereditary spherocytosis
- d) All of the above
Answer: d) All of the above
How is the osmotic fragility test performed?
- a) By placing red blood cells in a solution of increasing salt concentration and measuring the amount of hemoglobin released
- b) By placing red blood cells in a solution of decreasing salt concentration and measuring the amount of hemoglobin released
- c) By placing red blood cells in a solution of constant salt concentration and measuring the amount of hemoglobin released
- d) By placing red blood cells in a solution of varying pH and measuring the amount of hemoglobin released
Answer: a) By placing red blood cells in a solution of increasing salt concentration and measuring the amount of hemoglobin released
What is the principle of the osmotic fragility test?
- a) The principle of the test is to measure the level of hemoglobin in the blood
- b) The principle of the test is to measure the amount of salt in the blood
- c) The principle of the test is to measure the ability of red blood cells to resist osmotic pressure changes
- d) The principle of the test is to measure the pH level of the blood
Answer: c) The principle of the test is to measure the ability of red blood cells to resist osmotic pressure changes
What is the normal range of osmotic fragility in red blood cells?
- a) 0-20%
- b) 20-40%
- c) 40-60%
- d) 60-80%
Answer: b) 20-40%
What is the significance of increased osmotic fragility in red blood cells?
- a) It indicates a normal blood condition
- b) It indicates anemia or other blood disorders
- c) It indicates high levels of hemoglobin in the blood
- d) It indicates a normal blood cell shape
Answer: b) It indicates anemia or other blood disorders
What is the significance of decreased osmotic fragility in red blood cells?
- a) It indicates a normal blood condition
- b) It indicates anemia or other blood disorders
- c) It indicates high levels of hemoglobin in the blood
- d) It indicates a normal blood cell shape
Answer: a) It indicates a normal blood condition
Which of the following is an advantage of the osmotic fragility test?
- a) It is a simple and inexpensive test
- b) It is a test that provides immediate results
- c) It is a test that requires a large sample of blood
- d) It is a test that is not affected by other factors such as diet or medication
Answer: a) It is a simple and inexpensive test
